Our employees can work more efficiently because information is available to them faster. We are now able to provide much higher-quality information much faster across the board.
Edwin Mielke, IT Manager, Carl Fuhr GmbH & Co. KG
Solution: Quality management with enaio®
Quality management systems (QMS) are a key factor in long-term business success. The main advantages of such systems include transparency and information provided regarding processes as well as clearly defined organizational structures.
enaio® can be flexibly adapted to the application at hand, whether that be quality management in health care (administration, clinics, and hospitals) or in regulated environments such as in the pharmaceutical industry.
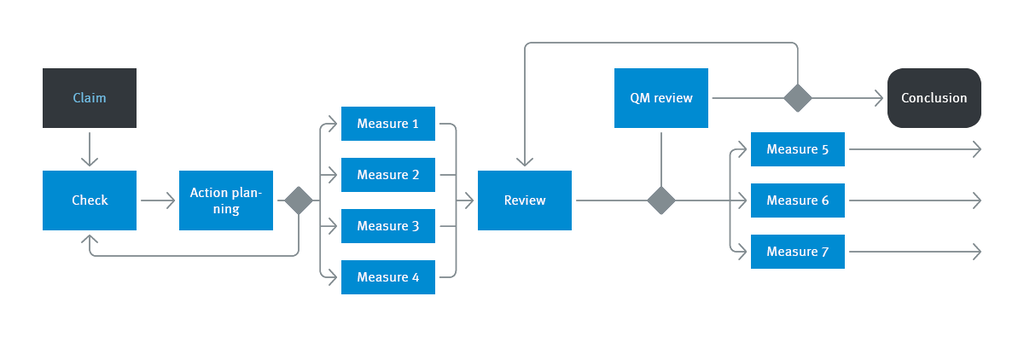
enaio® supports your business and work processes while harmonizing them in their entirety. The result is an information and process management tool where the individual modules and functions mesh together, from the recording of business processes to the mapping of complex workflows.
Map structured, value-creating processes electronically
Automatic document forwarding
End-to-end documentation of business processes
Standardized processes reduce employee workload
Information provided in the right context
Comprehensive change management and audit-proof archiving
Logging ensures the transparency of process workflows.
Electronic records provide information on the current status of events.
Missed deadlines are a thing of the
past.
End-to-end documentation and defined processes are two ways enaio® supports you in all your QM activities.
Quality management systems in use in the healthcare sector, in regulated environments, and in the technical field mean one thing in particular: end-to-end documentation. The requirements are often out of line with the costs associated with meeting them. ECM software such as enaio® allows you to automatically execute and secure document-based quality assurance processes. ECM functions, such as logged notices or subscriptions, improve the flow of information.
This allows you to integrate and use your specific QM and QA templates directly in enaio®. The documents are then automatically forwarded for further processing to other departments or employees, if necessary, using workflows. You are automatically notified of changes via a subscription service. Structured, standardized processes and automatic document creation, versioning, and distribution result in employees increasing their focus on value-creating processes.
enaio® helps you effectively manage a number of challenges related to market liberalization and the increase in the number of customers switching providers that comes with this, among other things. This allows for targeted customer loyalty measures and facilitates the selective provision of customer, contract, and other service information – directly via company portals, too.
Other EMC functions, such as digital customer records, technical documentation, comprehensive contract management, and e-mail administration, can help improve the quality of service and thus ensure long-term customer, supplier, and partner loyalty.
Bundle service information in electronic records
Provide the right information quickly
Improve your ability to provide information
Increase service quality and boost customer loyalty
Best-practice solutions for regulated environments
Optimally comply with regulations, guidelines, and laws
Comprehensive SOP, incident, and change management
Rapidly detect deviations and avoid errors
Transparent, clear documentation for quality processes